
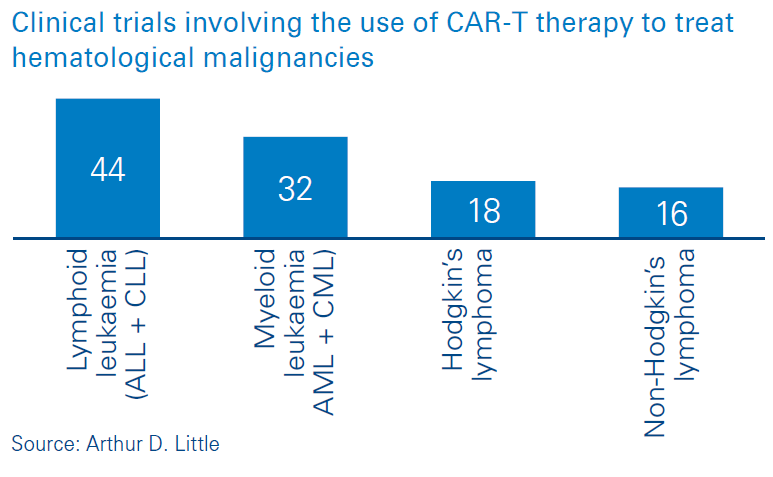
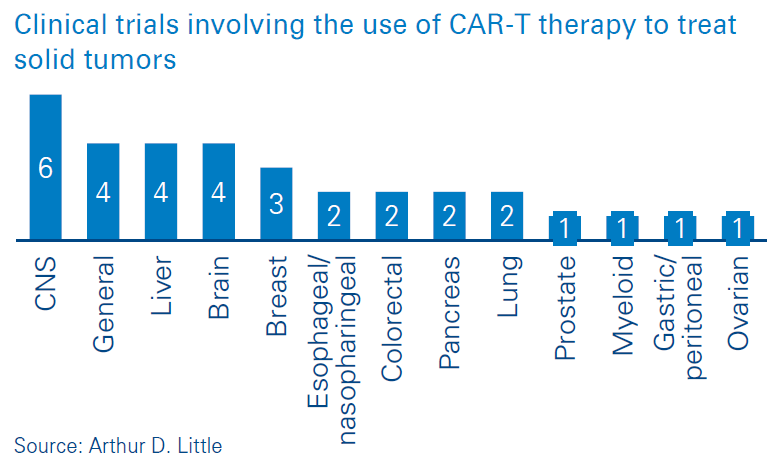
CAR-T (chimeric antigen receptor T-cell) therapy involves the use of genetically re-engineered versions of a patient’s own T-cells to find cancer cells and defeat them. As of March 2020, there are two CAR-T therapies licensed for use – Kymriah® and Yescarta®. CAR-T has pushed the boundaries of medicine, and the success observed in patient treatment has created interest in the use of CAR-T to treat a number of other types of tumors. This is evidenced by the number of clinical trials involving the use of CAR-T to treat a range of cancers (Figures below). Existing treatments require CAR-T cells to be manufactured remotely and transported to treatment centres for administration. Emerging technology now provides the possibility for CAR-T cells to be manufactured on site. In this viewpoint we outline the advantages of on-site CAR-T manufacturing.
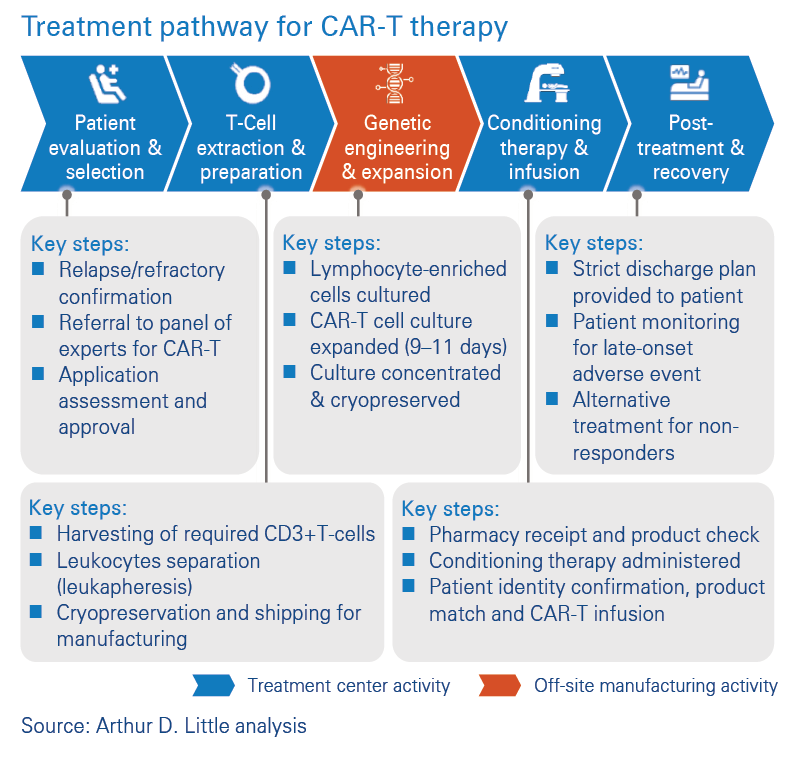
Existing approved treatments are based on off-site manufacturing
In a previous paper titled “Changing gears to deliver CAR-T in your hospital”, we outlined the treatment pathway for approved CAR-T therapies. With the current approved CAR-T treatments, the patient is evaluated and selected for treatment. Then their T-cells are extracted, leukocytes are separated, and the sample is transported to an off-site manufacturing facility, where the genetic engineering and cell expansion takes place. The final product is returned to the treatment facility for infusion into the patient, who would have received some coordinated conditioning therapy in preparation for the treatment. After treatment administration, the post-treatment plan is implemented to support the patient’s recovery. The logistical impact of off-site manufacturing is that the patient has to wait for an extended period of time for the cells to be processed and returned to the hospital before the treatment can continue. There are logistical risks associated with the transportation of samples, e.g., delayed arrivals, lost samples and sample deterioration. CAR-T therapy can be enhanced if the “extra mile” is eliminated without any impact on the quality of treatment.
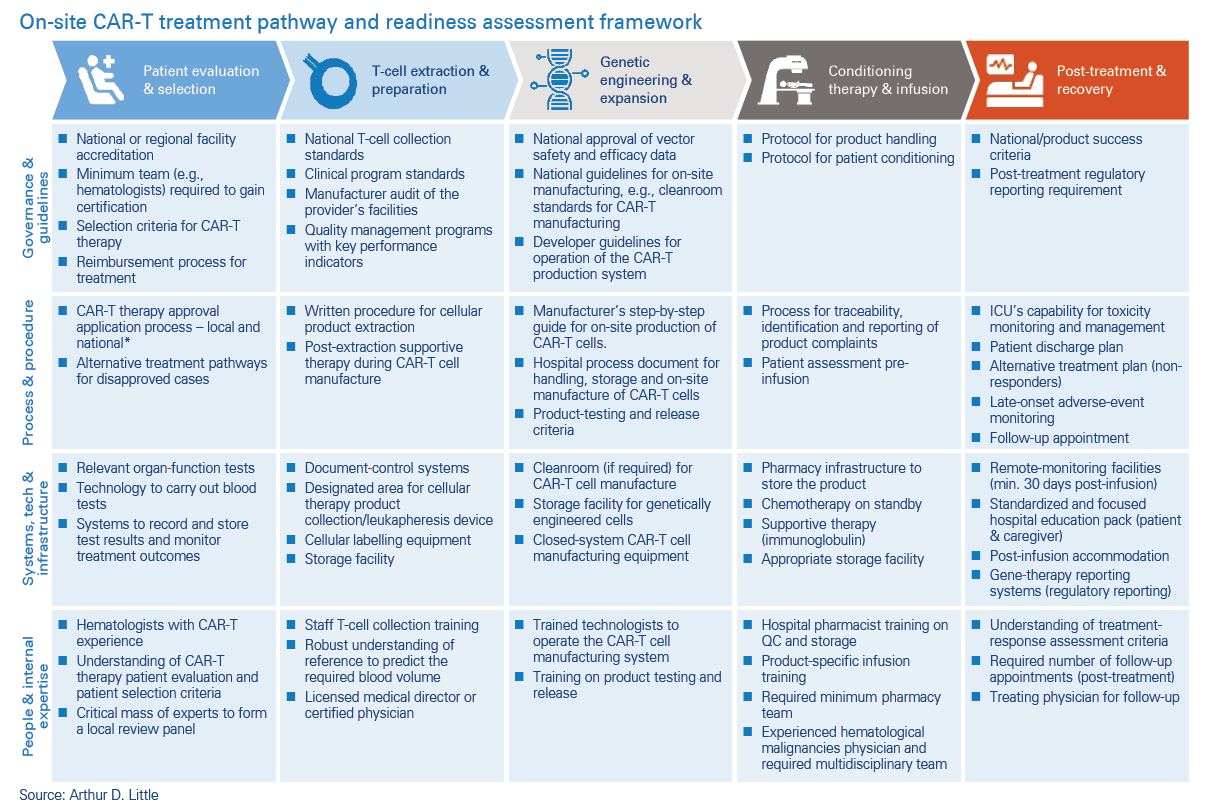
On-site CAR-T production is gaining momentum
As technology continues to evolve and more research is done with CAR-T, published papers that explore the possibility of carrying out every step of the CAR-T treatment pathway at the hospital have begun to emerge. The idea of this innovative move is to take advantage of the benefits of on-site manufacturing and improve patient treatment. It may present an opportunity for hospitals to offer an advanced and innovative treatment to patients. It also gives hospitals an opportunity to do clinical research in an area of cutting-edge medicine.
On-site production of CAR-T cells can deliver a number of potential advantages:
- Speed of availability – on-site production may be able to speed up the “vein to vein” time of patient treatment. One published study suggested that on-site-produced CAR-T could assist certain patients, who would otherwise not benefit from off-site manufactured CAR-T due to the rapid progression of their diseases.
- Wider access to treatment – on-site production may provide an opportunity for clinicians to consider CAR-T in a different light and enable wider access to this innovative treatment. This is because the availability of on-site manufacturing increases the independence of delivery by hospitals. This can be a game changer in countries that restrict movement of genetic materials and patient samples beyond their borders and do not have their own CAR-T manufacturing facilities.
- Ease of handling and improved cell viability – on-site production requires less process steps because some steps associated with off-site manufacturing, such as transportation and post-transportation thawing, will not be necessary. This will serve as an advantage for treatment. In a phase 3 trial that failed to meet its clinical end point, a review of the study suggested that thawing the cryopreserved cells may have contributed to the failure of the clinical trial. Another study suggested that the viability of the cells could have been compromised by vibrations during transportation.
The advantages of on-site manufacturing of CAR-T cells could increase its attractiveness to hospitals that may have considered CAR-T in the past but chose not to offer the treatment to patients for various logistical reasons. However, bringing the production of CAR-T in-house may present a number of extra responsibilities for hospitals and require effort (and even costs) that treatment using off-site-manufactured CAR-T cells does not.
Evaluation is the first step in considering on-siteproduced CAR-T therapy for your hospital
When considering on-site CAR-T therapy as a treatment to be delivered, it is prudent to first prepare an initial or outline business case to assess the opportunity. Subsequently, an operational readiness assessment of the hospital or treatment center may be necessary as part of a more detailed business case.
In carrying out an operational-readiness assessment, it is important to consider four key domains at each step of the treatment journey:
Governance and guidelines
This refers to the necessary accreditations, certifications and guidelines from the governing bodies and developers of the on-site manufacturing technology. It also involves establishing the governance required to assess and approve individual CAR-T therapy request applications. Some hospitals may have to form new panels of experts or augment the scope of existing suitable panels to provide the required governance. In some countries, governance may extend beyond local hospitals to regional or national review panels.
Processes and procedures
Processes are required to outline how different treatmentrelated activities should be carried out to ensure consistency and maintenance of high-quality standards. These include processes and procedures for selecting patients, handling extracted cells, testing and release of on-site manufactured CAR-T cells, managing post treatment side effects etc. These processes should be robust and subject to relevant GxP audits.
Technology and infrastructure
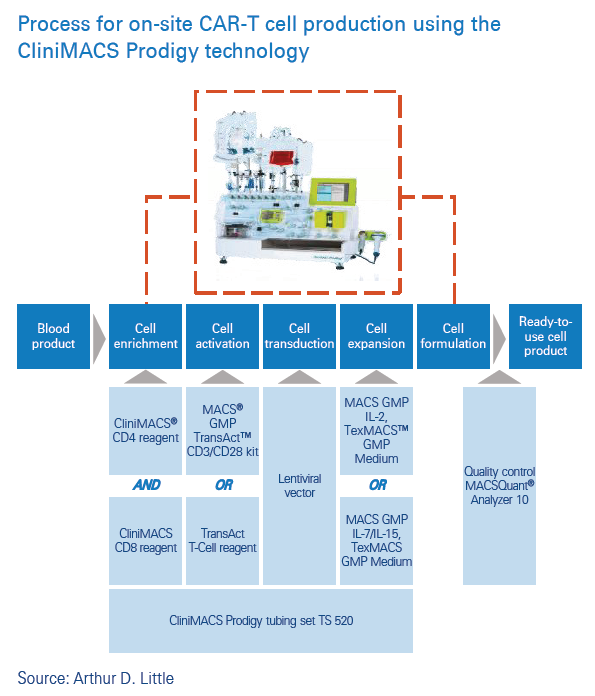
Technology and infrastructure play a central role in the delivery of CAR-T on site. They are needed to carry out the activities that precede genetic engineering and expansion of T-cells. These activities include running blood tests and harvesting the patient’s T cells. Technology and infrastructure are also required to manage the patient after they have been infused with the CAR-T cells. However, the most cutting-edge technology and infrastructure on the treatment pathway involve the set-up to produce the CAR-T cells on-site. The technology for this key step is available, with a number of clinical trials having been published in the last few years7,6. Setting up the technology and infrastructure for on-site CAR-T cell manufacture will require close collaboration with the developer company to support full implementation and compliance of on-site CAR-T cell manufacture.
Miltenyi Biotech has developed an automated manufacturing process for lentiviral gene modification and expansion of selected T-cells. The CliniMACS Prodigy® TCT (T-Cell Transduction) Process software allows purification and polyclonal T-cell stimulation, followed by gene modification and expansion of T-cells in a single-use closed-tubing set. Published studies suggest that the TCT process can handle highly diverse cell sources while yielding a consistent cellular product.
People and internal expertise
As with introduction of any new advanced therapy, additional expertise will be required to enable successful on-site manufacture and delivery of CAR-T therapy. As well as upskilling of existing staff, external personnel will be needed to provide expertise that is not already available within the hospital. Onsite- manufactured CAR-T cells will require strategic, technical and project management expertise to get the treatment center ready to deliver the treatment.
Get started with an evaluation for on-site CAR-T delivery in your hospital
CAR-T, a game-changing treatment, is available now but constantly evolving. More and more patients will request CAR-T therapy as it gains approval to treat more diseases. To be on the front foot, forward-thinking hospitals interested in CAR-T therapy should begin to examine the possibility of delivering it on site for the advantages this brings. It is a great time to consider delivering on-site CAR-T therapy because the data needed for marketing authorization are yet to be produced, hence, all the necessary preparations can be made in advance of a license being granted. If you want to deliver CAR-T therapy without the extra mile, you must begin an evaluation of on-site production of CAR-T for your hospital without delay.





