Patient recruitment drives success in clinical trial execution — but is increasingly difficult. To overcome this challenge and achieve wins, clinical trial sponsors must inspire study teams to beat clinical trial patient-recruitment timeline targets. This Viewpoint provides insights, case studies, and data outcomes of clinical trials that achieved accelerated patient recruitment and shows how this was accomplished.
According to legendary US college basketball coach Bobby Knight, the key is not the will to win … everybody has that. It is the “will to prepare to win” that is important. This will to prepare to win is becoming an increasingly crucial factor in clinical trial execution.
The key stakeholders in the healthcare system (i.e., patients, providers, payers, and drug developers) all benefit from a win in on-time clinical trial execution. Patients receive quicker access to new treatments, providers and payers gain innovative ways to meet their patients’ needs, and drug developers achieve a successful ROI for the risk taken by investors.
Clinical trial execution involves several activities, including site setup, patient recruitment, monitoring, data quality management, and so on. However, the most challenging aspect of on-time clinical trial execution is patient recruitment. Therefore, to achieve clinical trial acceleration, clinical trial sponsors must prevail in patient recruitment.
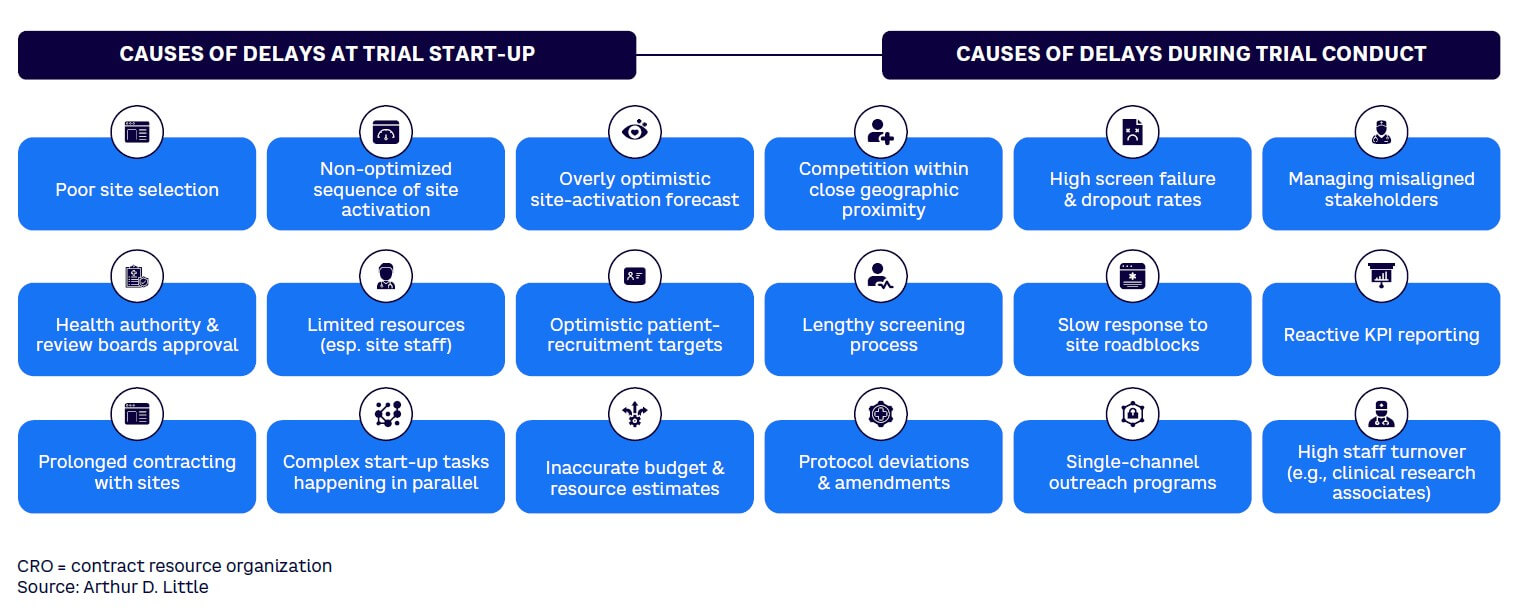
There are numerous reasons for slow patient recruitment in clinical trials (see Figure 1).
Winning in patient recruitment means addressing these barriers and beating the time expected to recruit the number of patients required to deliver a statistically meaningful result as well as addressing other requirements of regulators in target countries.
In some therapy areas, emerging treatments are curative, and the faster a sponsor completes the clinical trial, the faster it can gain access to existing patients in the market. Sponsors that are second or third in that race may have a limited pool of available patients, which may have very negative consequences on their commercial performance.
WHY THE GROWING CHALLENGE?
As more and more clinical trials are carried out every year, in many cases from a limited pool of potential patients, recruiting patients in those clinical trials is becoming increasingly challenging.
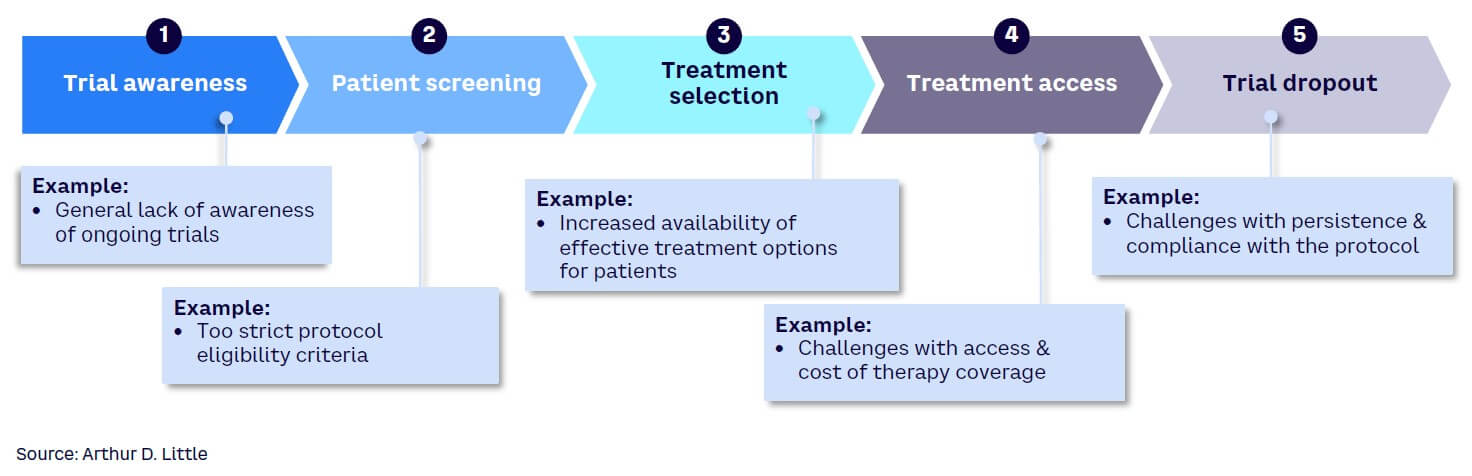
Several factors are responsible for the increasing challenge in recruiting patients for clinical trials. These reasons can be examined using the clinical trial patient treatment pathway as a guide, including the five key steps in the pathway (see Figure 2):
-
Trial awareness. Patients are rarely aware of ongoing clinical trials and hence may not proactively express an interest in participation. They usually rely on physicians for treatment-related information, and doctors may not always have clinical trials in the forefront of their minds as alternatives to propose to patients. Also, physicians may not be aware of the occurrence of a trial. Language barriers are also a factor that may drive lack of awareness of trials by patients. In addition, social and cultural barriers as well as mistrust of the medical system by some communities can impact the awareness of trials.
-
Patient screening. A patient may be identified as a candidate for a trial, but patient screening may reveal that the individual does not fulfill the requirements to be included. In some cases, the patient may hesitate to participate in the screening process if it is burdensome. This process can be very demanding and may be less appealing if there are more acceptable treatment alternatives available.
-
Treatment selection. Patients and their families may hesitate to opt for a trial even if there is a potentially significant benefit. Clinical trial treatments carry the risk that any unapproved treatment carries, and patients may prefer to try an approved drug with known benefits and risks over an experimental one, even when the experimental treatment may offer a higher potential benefit. With the growing number of approved treatment options available, the challenge with trial treatment selection is expected to increase.
-
Treatment access. In some countries where universal healthcare is not available, there may be access and affordability restrictions for those who may be ideal candidates for clinical trials. If a patient has no health insurance, for example, or the co-pay amount is high for certain treatments involving trials, he or she may be unable to participate. Many trials are held in academic healthcare centers, and some may be at a distance considered too far by patients to commit to regular visits. This is exacerbated by the increasing number of closures of trial sites in some established clinical trial countries due to lack of financial viability, requiring patients to travel farther afield to access the trials. Furthermore, many regulatory authorities require lifetime access to the treatment for patients who participated in the trial if the treatment is successfully approved.
Trial sponsors that cannot afford these requirements will avoid countries with such requirements even if many potential patients reside there.
-
Trial dropout. Clinical trials usually require a lot of visits to healthcare facilities, particularly in non-decentralized trials. For patients who have busy lives or may not have long to live, it may be challenging to keep them in the trials for a long time. Patients may, at some point, consider it more important to attend to pressing domestic priorities or spend the brief time they have left with loved ones rather than make recurring visits to a healthcare facility. This may lead to dropout.
To win with clinical trial execution, sponsors must carefully consider why patients may either fail to enroll or choose to drop out of trials.
HOW TO WIN
While there have been many efforts to describe ways to accelerate clinical trials, many trial sponsors struggle with the “how” question: how do you speed up a clinical trial? Therefore, our focus in this Viewpoint is on how to prepare to win using six effective steps in trial execution.
1. Shift mindset from “patients as targets” to “patients as partners”
Many trial sponsors still see patients as targets to meet recruitment numbers rather than partners on the winning journey. When patients are considered key partners, clinical trial sponsors will prioritize patients’ needs and remove every obstacle to participating while using all possible means to incorporate their preferences throughout the trial journey. While most major clinical trial sponsors claim to be patient-centric, it is not uncommon for the patient’s voice only to be heard just once at the start of the trial. A shift in this mindset requires continuous listening, review, and prioritization of patients’ needs throughout the trial journey.
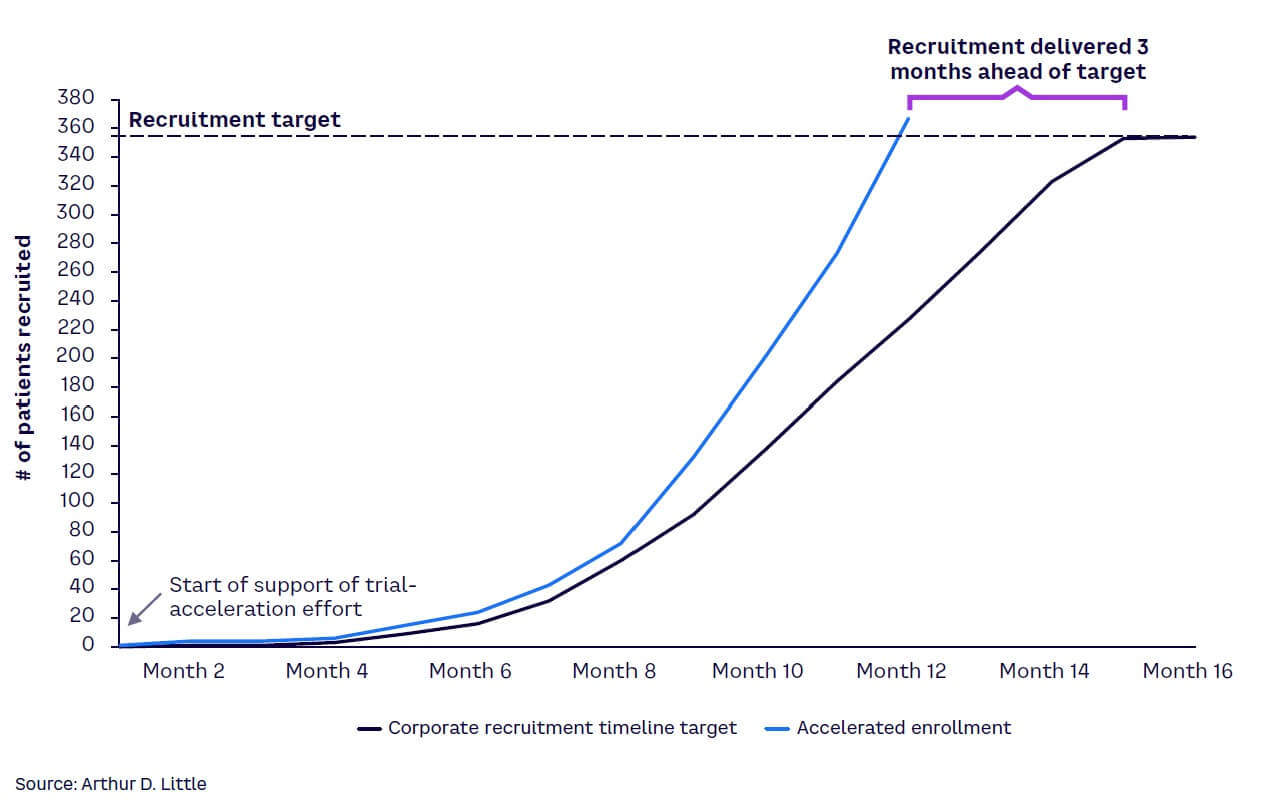
The case study in Figures 3 and 4 illustrates an example where real-time insights helped increase patient recruitment. By mapping out a patient’s journey and considering their personal costs and emotional response at each stage on an ongoing basis, sponsors can assess the patient’s needs and treat them as their most important partner.
This may include using generous financial measures to ensure the patient does not suffer any loss — from covering the costs of transportation, child and pet care, patient companions, and accommodation to providing some relief for working patients concerned about loss of income due to participation.

2. Avoid hearsay-driven decision-making & follow data instead
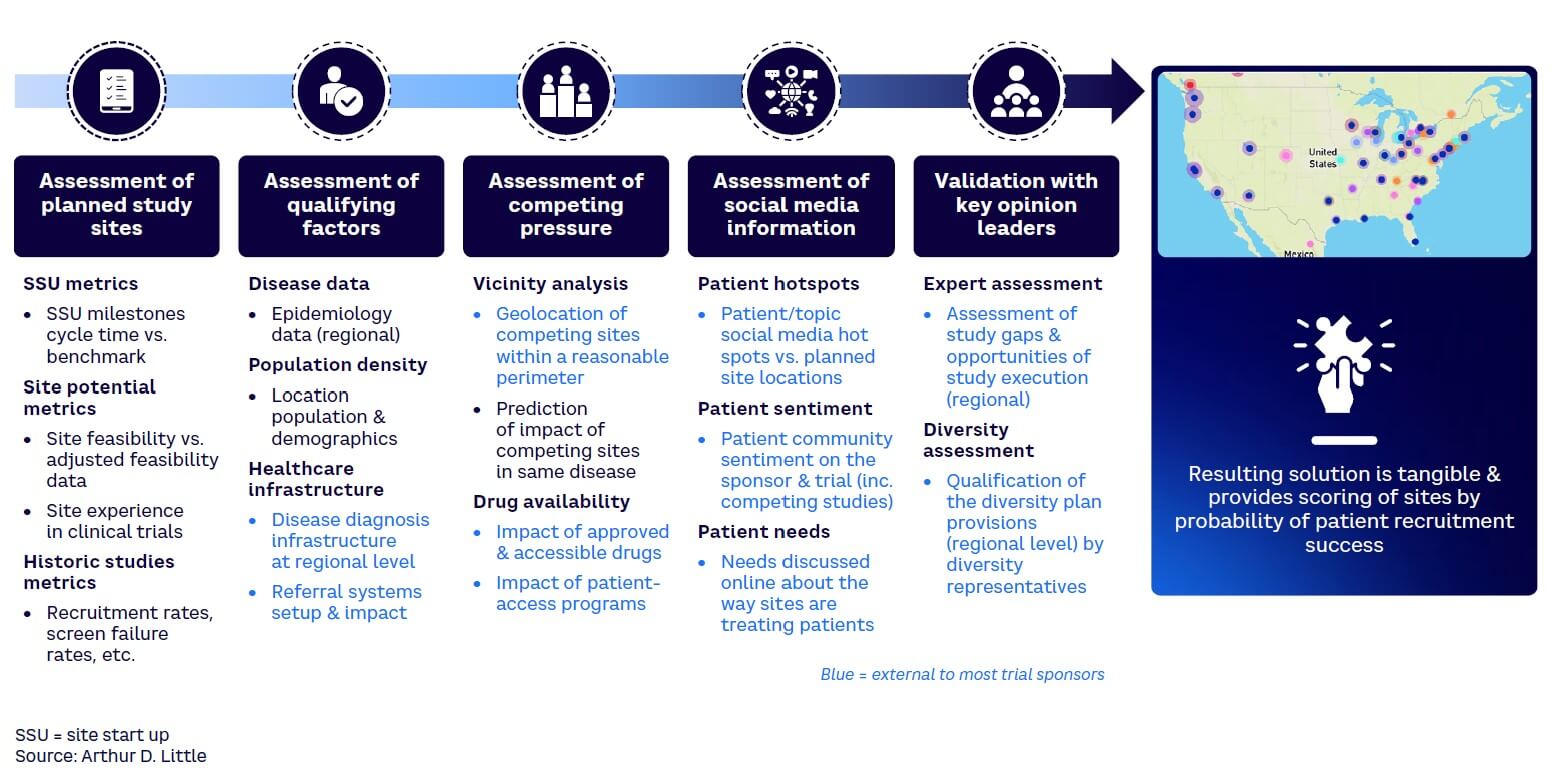
In many trials, data sits idle while people make hearsay-based decisions. At every step in the trial process — from concept to design and execution — there is siloed data that can be triangulated to gain better insights and make smarter decisions. This may include combining external-to-trial-sponsor data with very granular site-level data to make decisions related to a targeted engagement rather than using a standard approach across all sites. Predictive intelligence can speed up trial execution if a connected data-driven approach is pursued in answering the main exam questions. Across all stages of the trial journey, there is plenty of data available to improve decision-making for those prepared to make the extra effort. Assessments at a granular level also enable a sponsor to identify trends and good practices to leverage across all relevant sites. As an example, Arthur D. Little (ADL) used traditional site start-up data with other data (e.g., patient community sentiment, optimal diversity index, Google mobility data) to find some of the highest-performing sites on a rare disease clinical trial (see Figure 5). This helped reduce trial time by several months.
3. Encourage technophile mindset & deploy latest technology
Technology changes quickly, providing more ways to win in clinical trial execution; for example, AI-enabled solutions to rapidly review large volumes of real-world data for patient matching, utilizing social media intelligence to find patient hotspots and prescreening patients, and applying graph networks to identify potential pre-diagnosis patients. With the speed of new technology introduction, we have seen several cases where the solutions used in trial execution nearly become obsolete in the time between trial design and trial close. Several use cases of technology-enabled trial acceleration exist that show that technology deployment in clinical trials can help gain execution speed even if it requires a costly protocol amendment. Experience suggests that clinical trial teams can be reluctant to embrace recent technology. However, it takes a technophile mindset and a desire to continuously search out and deploy these tools to enable success in clinical trial execution.
4. Know competing trial protocols better than your own
Successful sales representatives are well-versed in identifying the strengths and weaknesses of competing offerings and know how to position the value of their offerings to meet customer needs. Applying this mindset in clinical trials can enable trial sponsors to prevail. It is important to first understand the challenge that the active drug in your clinical trial addresses in the treatment landscape and then contrast this with competing offerings in clear communications by all roles with contact of patient-access groups, principal investigators, and other stakeholders. It is also vital to have a deep understanding of the site and patient offerings provided by competing trials and strive to match or outperform those offerings. Figure 6 illustrates the potential of using competition to increase recruitment.

5. Anticipate risks & opportunities using leading indicators; take prompt action
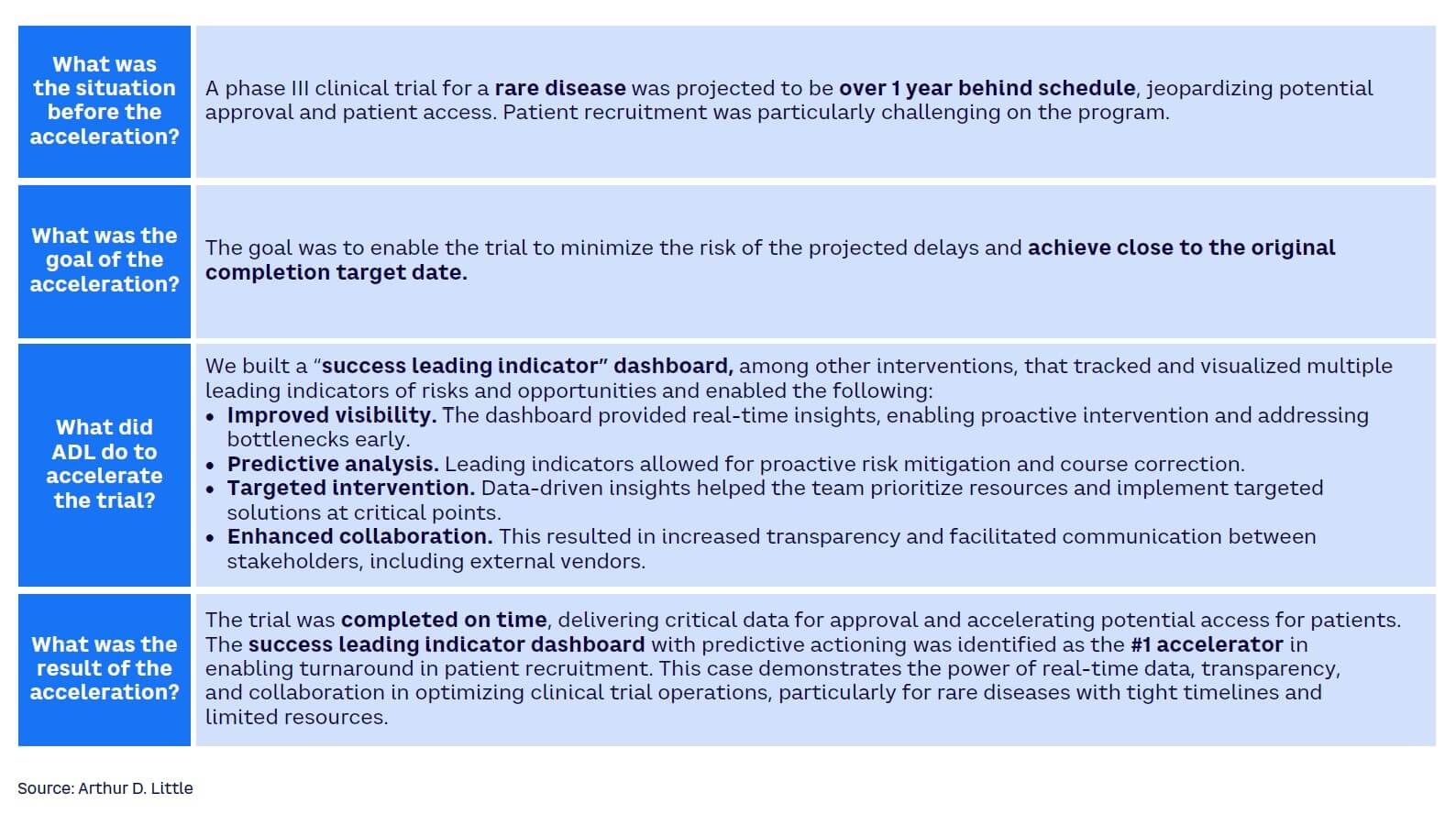
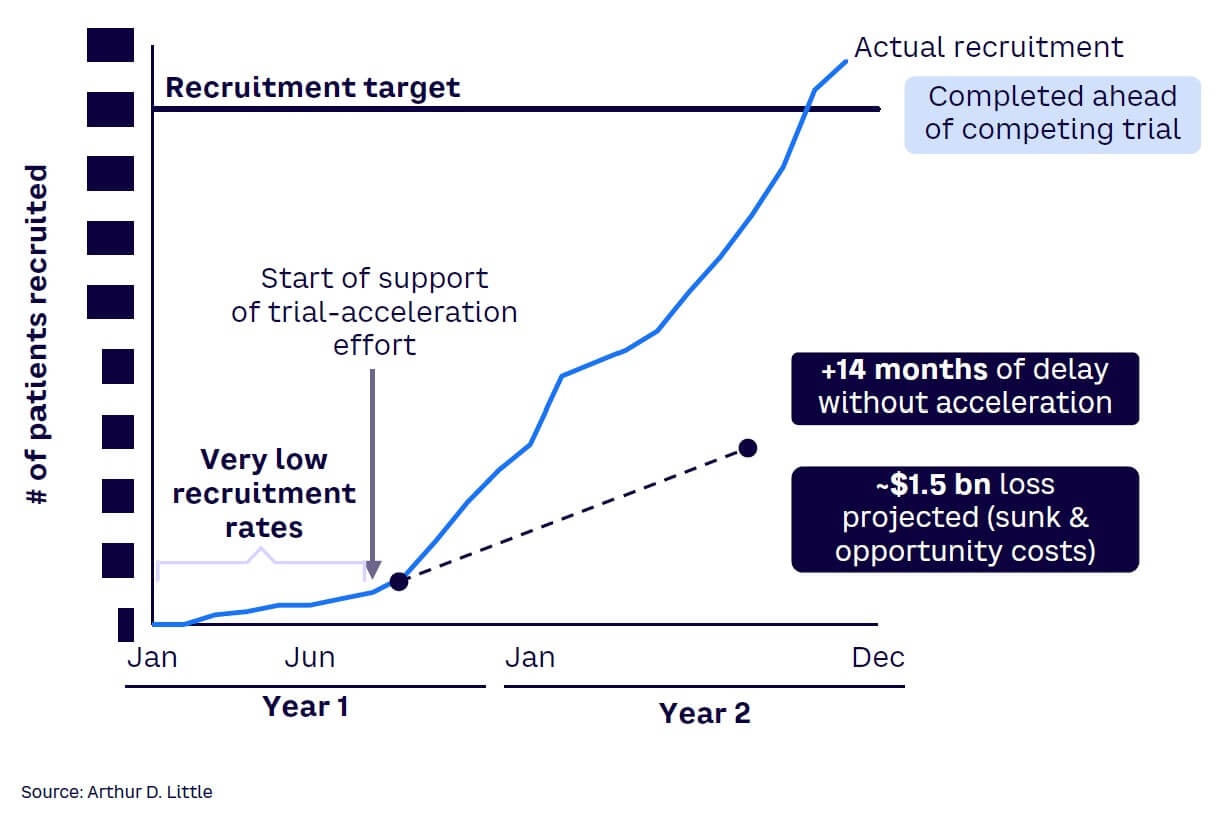
Many clinical trial teams watch helplessly as the trial goes through a protracted and agonizing struggle to take off. They rely on outdated information or lagging indicators to make decisions and cannot anticipate challenges before they become serious. Leading indicators can help anticipate risk and support winning clinical trial teams to seize emerging patient-recruitment opportunities. In a global phase III trial, ADL designed a “one-source-of-truth leading indicator dashboard” to predict and anticipate risk (see Figures 7 and 8). In the post-trial review, the sponsor considered the dashboard as the highest contributor to the early completion of patient recruitment in the trial. Achieving the benefit required the connection of data from various data sources, applying the key risk/opportunity indicator methodology, and making the intelligence available to all trial team members, including the supporting contract research organization and other vendors.
6. Establish senior management sponsorship
To win in clinical trial execution, a dedicated focus must exist at very senior levels in the organization, and all available resources must be employed to remove barriers and drive acceleration. Unfortunately, many senior leaders are too distanced from the realities on the ground, relying on monthly progress reports or budget requests to understand the situation with their clinical trials. While it is not possible for senior leaders to examine all clinical trials, it is important that they dedicate attention to the clinical trials that are vital to the future of the company. For the things that really matter and the lifeline of any company, unison between the “doers” and the business leaders in addressing challenges will catalyze acceleration. Conversely, insufficient senior leadership involvement will adversely impact the study and the team’s ability to win.
Conclusion
WINNING IN CLINICAL TRIALS: DO YOU HAVE WHAT IT TAKES?
Winning in clinical trial execution is beneficial to all key stakeholders, as it enables sponsors to maximize the rewards from years of investing time, money, and know-how. Furthermore, with the increasing shift to curative therapies, second and third movers to launch may find they have far fewer patients to treat in the future. Yet, even with a will to win, many sponsors struggle as challenges prevent seamless patient recuitment. It thus falls to sponsors that have the will to prepare to win to be bold and shift the mindset to see patients as partners, following the data in making decisions, using the latest technology to maximum effect, knowing competitor protocols better than yours, anticipating danger using leading indicators coupled with quick action, and devoting senior leadership attention to trial acceleration.
By winning in on-time clinical trial execution, the entire healthcare system benefits, giving patients better access to treatments, providers and payers a better opportunity to meet their patients’ needs, and pharma/biotech companies receive a higher ROI. With clinical trial execution, winning does not come to those who wait.



